January 2020 update
After the January/February 2020 issue went to press in December, the U.S. Department of Health and Human Services (HHS) updated the HIV treatment guidelines.
Dolutegravir is now a preferred medication in pregnancy as well as an alternative drug for those who are trying to conceive; go to aidsinfo.nih.gov/guidelines/html/3/perinatal-guidelines/0. This clears preliminary concerns over the potential for birth defects with dolutegravir, raised in 2018 by a study in Botswana.
The U.S. Food and Drug Administration (FDA) updated the dolutegravir drug label in October 2019, based on final results of the Tsepamo Study in Botswana.
The FDA said there were five cases of neural tube defects (NTDs) reported out of 1,683 deliveries (0.3%) among women exposed to an HIV regimen containing dolutegravir around the time of conception. The popular HIV antiretroviral is sold under the brand name Tivicay, and is found in Dovato, Juluca, and Triumeq.
In comparison, the rate of this birth defect was lower for women with HIV who were not taking dolutegravir (0.1%; 15 out of 14,792 deliveries). But it was higher for HIV-negative women (0.8%; 70 out of 89,372 deliveries).
The Baylor College of Medicine reported last July on a laboratory study conducted with dolutegravir following the preliminary findings in Tsepamo. As has been known for a long time, the Baylor researchers found that the nutritional supplement folate acid protects embryos against NTDs.Go to bit.ly/2YAbCjA.
The FDA advises against taking dolutegravir around the time of conception and throughout the first trimester. Switching to a different regimen during the first trimester would depend on a number of considerations. Read the FDA announcement at bit.ly/2O9D7KP.
New HIV med on the way: fostemsavir
ViiV Healthcare submitted a New Drug Application (NDA) to the Food and Drug Administration (FDA) for fostemsavir, a medication for adults with extensive HIV treatment experience who have multidrug-resistant HIV and are unable to form a suppressive regimen due to resistance, intolerance, or safety considerations.
Fostemsavir has been granted FDA Fast Track and Breakthrough Therapy Designations. A breakthrough designation requires preliminary clinical evidence indicating that the drug may demonstrate substantial improvement on clinically significant endpoint(s) over available therapies.
Fostemsavir is an oral attachment inhibitor that works differently than another currently on the market, Trogarzo. See the fostemsavir page in the Positively Aware HIV Drug Guide: positivelyaware.com/drug-guides/fostemsavir.
“Fostemsavir may provide an important treatment option for the group of people living with HIV who, for a variety of reasons, are not able to suppress their virus with other medicines and could be left with few or no treatments available to them. In keeping with our mission of leaving no person with HIV behind, we have overcome many barriers to bring this important new medicine to people living with HIV, including investing in what is a very complex manufacturing process,” said Deborah Waterhouse, ViiV’s CEO, in a December 2019 press release announcing the NDA. The company is solely devoted to HIV therapy and cure.
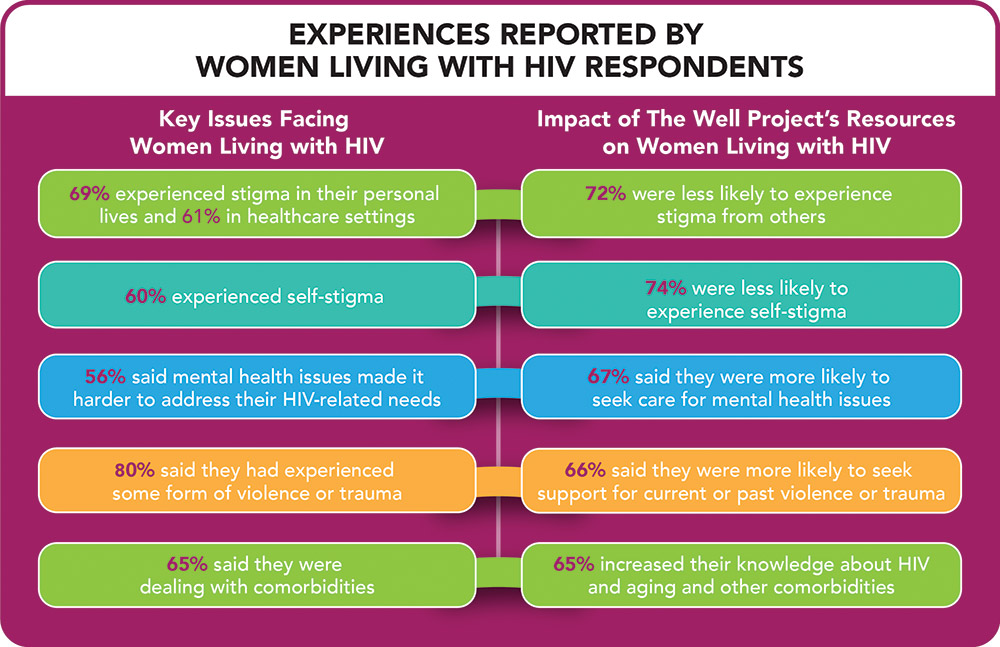
Results of a new survey conducted by The Well Project offer insight into the experiences and needs of women living with HIV. The report, Together We Are…Making an Impact, highlights the need to look beyond viral suppression (undetectable viral load) as an indicator of quality of health.
The report is the result of a survey of 239 respondents, two-thirds of whom identified either as cisgender or transgender women living with HIV, and points out that there remain numerous opportunities to improve the lives of women living with HIV, including:
• Implementing educational programs to reduce stigma experienced by women living with HIV in healthcare settings and their personal lives
• Normalizing mental and behavioral health issues, including current or past violence and trauma, and encouraging women living with HIV to seek the care they need
• Emphasizing the need for education for healthcare providers about the stigma-reducing messages of U=U (Undetectable Equals Untransmittable)
• Driving research on and increasing attention to the numerous issues around living with HIV over the long-term (including co-morbidities)
• Continuing to promote opportunities for women living with HIV to connect with one another
Established in 2002, The Well Project is a leading resource for women living with HIV: thewellproject.org.
Mail order testing yields results
Free home HIV testing kits provided through mail order to men who have sex with men (MSM) offered an effective strategy for getting people into care, according to a study conducted by the Centers for Disease Control and Prevention (CDC).
Using banner ads on social media and music websites, 2,665 men were recruited for the 12-month-long study. Half were sent four free HIV testing kits, with the option to order more—many of them did, sharing the kits with friends. The other half only got a link to local testing services. All participants completed quarterly surveys and had access to online HIV resources and telephone counseling upon request.
Among those who received testing kits, 25 men learned they were living with HIV, as did 11 from the other group. And from the home tests shared with friends, another 34 men were diagnosed.
More than 70 percent of participants who tested positive sought treatment.
“Self-testing is an important option for some people and in some situations, it saves time, offers privacy, and reaches people who may not be able to or willing to access existing testing services,” said Robin MacGowan, the study’s lead author and a researcher at the CDC in a statement.
The study used the OraQuick saliva test, the only approved and available over-the-counter home HIV test, and a finger stick blood test, which was sent to a lab. The men were given up to $90 for participating if they reported test results and completed the surveys.
New York City, Los Angeles, and the states of New York and Arizona are among those that have home testing programs. Arizona’s program is administered by Aunt Tina’s Foundation, a nonprofit based in Phoenix.
Home testing helps extend access to large rural areas, but is also useful for people living in urban areas and the suburbs, said Glen Spencer, executive director of Aunt Tina’s. “It’s also helpful in communities of color, where stigma can be more pronounced,” he added. “Many aren’t comfortable from a cultural standpoint in going to an HIV testing facility, but still want to know their status.”
In addition to the home tests, Aunt Tina’s provides online resources statewide. People who test negative can get information about PrEP. Arizona resources are available at hivaz.org, and in Spanish at vihaz.org. After taking an online survey, Arizona residents can order a free home testing kit at mysterykit.org, or order in Spanish at mipaquete.org.
Second reported case of HIV transmission during vaccine study
A second case of HIV transmission to a partner from someone who had temporarily stopped their HIV treatment to participate in a cure study has been reported. The report has renewed discussion about whether HIV-negative partners of people in cure studies should be offered PrEP.
The search for an HIV cure involves analytical treatment interruptions (ATIs)—it’s the only way to determine if a potential strategy will work. People living with HIV participating in a cure study may agree to an ATI, temporarily going off their HIV medication so that researchers can see if the virus becomes detectable again, how quickly, and at what viral load.
The first case of a sexual partner acquiring HIV during a cure study took place in France in November 2014, and was first reported in February 2019 in the Journal of Infectious Diseases.
A second reported case of ATI-related transmission occurred in Spain during a study that started in 2016 and concluded in May 2019. The study participant was a gay man in his forties who had been diagnosed with HIV 12 years earlier, and on suppressive therapy for four years. He became detectable four weeks into the ATI after reporting symptoms similar to acute HIV infection, and was restarted on his previous ART regimen four weeks later.
Prior to the partner’s ATI for the study, the couple had been counseled on HIV prevention methods, including condom use and PEP (post-exposure prophylaxis). At the time, PrEP was not available in Spain, but could be purchased online. PrEP did not become widely available in Spain until November 2019.
In an interview with the British HIV website aidsmap.com, study co-author Dr. Lorna Leal of Barcelona General Hospital said, “finding the best option to prevent secondary transmission is mandatory, since ATI remains the most frequently recommended intervention to analyse the impact of cure-related strategies.”
This report drew from a number of online sources, primarily from aidsmap: bit.ly/2pNT687.
Court clears way for safe injection site
After political struggles that climaxed in a court battle, Safehouse, the first safe injection site in the United States was set to open in Philadelphia. As this issue went to press, the facility was scheduled to open on January 1, pending a final ruling.
Available in other parts of the world, these sites allow people to use drugs under the supervision of trained harm reduction personnel to help prevent or treat an overdose. Additional services would include on-site initiation of Medically Assisted Treatment (MAT), recovery counseling, education about substance use treatment, basic medical services, and referrals to support services such as housing, public benefits, and legal services.
Co-founders Ronda B. Goldfein, executive director of the AIDS Law Project of Pennsylvania, José A. Benitez, executive director of Prevention Point, a local nonprofit, and Ed Rendell, the former Democratic governor of Pennsylvania, wrote an essay about the need for places like Safehouse, which appeared October 15 in the Washington Post: bit.ly/Safehousecommentary.
Julie Rhoad, CEO of The Names Project, and National AIDS Memorial CEO John Cunningham At the Library of Congress, announcing the transfer of the AIDS Quilt and its archives. (photo: Shawn-Miller, Library of Congress)
The 50,000-panel National AIDS Memorial Quilt is headed to the Bay Area under an agreement that hands over care and management of the quilt from the Names Project to the National AIDS Memorial Grove in San Francisco.
In a report by the Bay Area Reporter (BAR), Names Project CEO Julie Rhoad, president and CEO of the Names Project, told the publication that the nonprofit will transfer its operations to the AIDS grove and that The Names Project "will close and cease operations," shutting down by the end of the year.
The Names Project was established in 1987 in San Francisco as custodian of the quilt, which was conceived in memory of people who had died as a result of HIV/AIDS. In 2001, the nonprofit moved to Atlanta, along with the quilt. The agency had also organized various displays and educational events across the country and throughout the world.
The quilt will be relocated early this year to a warehouse near Oakland International Airport, said AIDS grove CEO John Cunningham in an interview with the BAR. The AIDS grove plans to build a new facility in San Francisco, the Center for Social Justice, which will house the quilt.
An archive of more than 200,000 items—personal letters, photographs, and biographical records related to people memorialized in the quilt, along with a sewing machine that was used to stitch together part of the quilt—will be contained at the Folklife Center of the Library of Congress, in Washington, D.C.
Comprised of more than 50,000 sewn panels measuring 3 feet by 6 feet, honoring 105,000 people, the quilt weighs 52 tons.
More information: AIDSmemorial.org and LOC.gov.
Drug label update
Efavirenz and nervous system toxicity
The FDA in October added information about neurotoxicity to the drug labels of medications containing the HIV antiviral efavirenz—Sustiva, Atripla, Symfi, and Symfi Lo. The FDA said neurotoxicity may occur months to years after starting therapy with efavirenz. Called “late-onset neurotoxicity,” adverse events include ataxia (poor coordination) and encephalopathy (disease, damage, or malfunction of the brain). The agency said the effects have been seen in people who have a genetic trait called “CYP2B6.” This trait is associated with increased levels of efavirenz despite standard dosing.
“Patients presenting with signs and symptoms of serious neurologic adverse experiences should be evaluated promptly to assess the possibility that these events may be related to EFV [efavirenz] use, and whether discontinuation is warranted,” the FDA reported.
Read the FDA announcement: bit.ly/37sK8Ow.
OI GUIDELINES Update
Kidneys, pneumonia, and more
The U.S. guidelines on opportunistic infections (OIs) in HIV were updated in October and November.
Tables relating to medications for OIs were updated, including dose adjustments needed for people with kidney problems.
Key changes were made to the section on community-acquired pneumonia.
The section on talaromycosis has been updated. Read “What’s New in the Guidelines”: bit.ly/updatedHIVguidelines2019-11-21.
Briefly plus
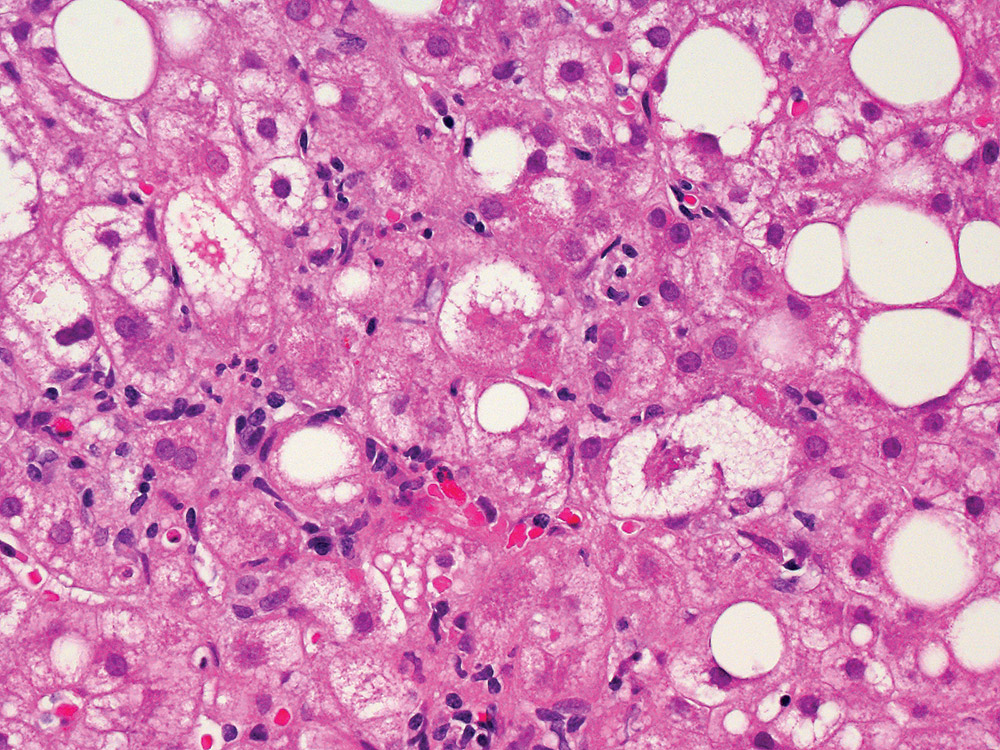
Liver tissue affected by non-alcoholic fatty liver disease (NAFLD). The large and small white spots are excess fat droplets filling liver cells (hepatocytes). (photo: Dr David Kleiner, National Cancer Institute)
by Julie Taddeo
Tesamorelin (Egrifta) may be an effective agent for reducing liver fat and preventing fibrosis in people living with HIV, according to a study released in October by the National Institute of Allergy and Infectious Diseases (NIAID) and the National Cancer Institute.
Tesamorelin, an injectable growth-hormone-releasing synthetic hormone, was approved in 2010 by the FDA to reduce visceral fat in people with HIV. A potential complication of HIV, antiretroviral therapy, or both may be changes in the distribution of adipose tissue (fat), otherwise known as lipodystrophy. Abdominal lipohypertrophy (a form of lipodystrophy) is the accumulation of excess visceral adipose tissue (VAT)—deep belly fat surrounding the liver, stomach, and other abdominal organs. Visceral fat is directly associated with liver inflammation and fibrosis, which can help lead to non-alcoholic fatty liver disease (NAFLD). “Because tesamorelin proved effective in treating abnormal fat build-up in the abdomens of people in the context of HIV and related medication use, we hypothesized that the drug might also reduce fat that accrues in the liver and causes damage in a similar population,” explained one of several lead investigators, Steven K. Grinspoon, MD, chief of the Metabolism Unit at Massachusetts General Hospital.
NAFLD affects 25% of people living with HIV in the developed world. “Many people living with HIV have overcome significant obstacles to live longer, healthier lives, though many still experience liver disease,” said NIAID director Anthony S. Fauci, MD, in a press statement. “It is encouraging that tesamorelin, a drug already approved to treat other complications of HIV, may be effective in addressing non-alcoholic fatty liver disease.”
Of the 61 participants from the double-blind, randomized study, 43% had at least a mild case of fibrosis and 33% had the more severe subset of NAFLD called nonalcoholic steatohepatitis (NASH). Participants were randomly divided into two groups: 31 received 2 mg injections of tesamorelin daily and 30 were given placebo injections. All participants were given nutritional counseling and instruction on how to self-administer the injections. Liver health was examined in both groups at baseline and 12 months later.
Participants who were given tesamorelin had better liver health than those who received the placebo. A healthy hepatic fat fraction (HFF, or fat to liver ratio) was achieved by 35% of the participants who received tesamorelin, while only 4% of those who were given placebo were able to do the same with nutritional advice alone. Additionally, nine placebo participants experienced onset or worsening of fibrosis, while only two from the tesamorelin group reported the same.
According to the study results, tesamorelin reduced the risk of developing NAFLD by 37%. Aside from joint pain, skin irritation, and stomach pain around injection sites, the drug was well tolerated overall. Several blood markers associated with inflammation and liver damage (including the enzyme alanine aminotransferase) also decreased more among participants taking tesamorelin, specifically those who had increased levels upon entering the study.
Investigators suggest that the indication for tesamorelin be expanded to include people living with HIV and diagnosed with NAFLD. They recommend that further research into the long-term effects of tesamorelin as protection against serious liver disease in people without HIV. “Our hope is that this intervention may help people living with HIV, as well as benefit HIV-negative people with liver abnormalities,” said Colleen M. Hadigan, MD, senior researcher at NIAID’s Laboratory of Immunoregulation.
SAGE conference focuses on LGBT senior housing
Elderly members of the LGBT community face far more barriers finding adequate housing than their heterosexual counterparts, according to SAGE, an advocacy and services organization for LGBT seniors. SAGE reported that when applying for housing, 48% of same-sex couples are subjected to discrimination. This was the focus of a SAGE conference. Sponsored by Citi Community Development, the conference was held in Washington, D.C. at the National Community Reinvestment Coalition. The event brought together nonprofits, housing developers, and policy experts from around the country to discuss LGBT housing and to collaborate on strategies to combat the disparities within it.
Finding safe, affordable housing is a common concern for many older people; addressing that challenge, while also facing discrimination from property owners, residents, and company representatives, can make the entire process feel futile. In a press release, Bob Annibale, Global Director of Citi Community Development, said that, “The LGBT population age 50 and above is expected to grow from three to seven million over the next decade, yet there are only about 600 units of affordable housing that are equipped with the appropriate training and services to meet their unique needs.” Additionally, there are currently 26 states with laws which allow property owners and providers to evict or refuse prospective residents based on who they are or who they love.
This conference was held as a part of SAGE’s National LGBT Elder Housing Initiative. By creating a platform for housing experts and LGBT advocacy organizations to exchange information and ideas, SAGE hopes to establish new partnerships and identify collaborative solutions to ensure that elderly LGBT members have improved access to culturally competent housing.
Charles Sanchez plays Merce, the title character of the web series he co-created.
Put off by the outdated and tragic portrayals of HIV often depicted in the media, Charles Sanchez, a writer/performer living with HIV, attempts to break the misconceptions with his award-winning musical web series, Merce. The show centers on the adventures of Merce, a single, middle-aged, gay man living with HIV in New York City, and comically follows his journey to find love and self-acceptance. What makes the show unique is Merce’s optimistic, cheerful, and energetic attitude, proving that, “life can be positive, even when you’re HIV+.” The show is intended to inspire and represent the modern life of a person living with HIV.
The second season of the series has been renewed by Skipping Boyz Productions and is scheduled to launch January 2020 with eight new episodes. Director Tyne Firmin shares how the success of the first season allowed them to raise the production budget for the second. “I think the audience is going to love the hilarious world we’ve created,” Frimin exclaims. Two new composers, Rob Hartmann and Adam J. Rineer, will also be joining the production crew. Season two will continue its bawdy humor while addressing important topics such as serodifferent relationships, comorbidities, U=U, PrEP, stigma, and marriage equality.
In anticipation of the release, producers are leaking musical numbers from the second season. Listen to music from the first episode composed and written by Rob Hartmann: bit.ly/348TVqS. Watch the second season of Merce: bit.ly/2XLt028.
Reflections from our intern
As I near the end of my internship, I leave Positively Aware having met so many people living with HIV with a renewed and deeper appreciation of life. I’ll admit, as a 24-year-old, it’s easy to get caught up in day-to-day trivialities.
But the gratitude for life that I have seen among people with HIV is inspiring. Many find purpose in community involvement or advocating for something larger than themselves. These are principles that I too can find value in pursuing. —JT
Viral Hepatitis Briefly
by Andrew Reynolds
Hepatitis D drug development guidance
The FDA has announced the draft guidance for development of antiviral drugs to treat chronic hepatitis D (HDV). Hepatitis D, also called “delta hepatitis,” can only be acquired by people who have hepatitis B (HBV); HDV cannot replicate itself without HBV. HDV is transmitted from blood-to-blood contact, and can be either acute (short-term) or chronic (long-term). To date, there are no treatments nor is there a vaccine for HDV. Overall, it is rare in the United States; according to the CDC, there’ve been approximately 357,000 people with past or chronic HDV, but rates of infection are higher among Asian immigrants and other foreign-born individuals where both HBV and HDV are more endemic. HDV can lead to serious liver problems such as cirrhosis, liver failure, and liver cancer. Developing treatments and cure for this overlooked disease is a very important intervention, and the guidance and support provided by the FDA is an important first step toward a cure. Announcement of the draft guidance came November 1. Source: bit.ly/HVDdraftguidance.
Baraclude treatment updates for hepatitis B
Changes to the label of Baraclude (entecavir), an antiviral used for treating chronic hepatitis B (HBV), were approved by the FDA on November 8. The changes are the result of a five–to-10-year observational study of 12,378 people, in which approximately half took Baraclude, and the other half took other HBV medications. Patients were monitored every six months, focusing on a variety of clinical issues including malignant tumors (neoplasms), liver-related HBV disease progression, liver cancer, and death. The study found that Baraclude was not associated with increased risk of tumors when compared with other HBV medications. Similarly, Baraclude was not associated with lower rates of HBV-related disease progression or death when compared to other HBV treatments. To date there is no cure for HBV, and while not everyone with chronic HBV needs treatment, research like this helps patients and medical providers make important choices about which treatment is right for them.