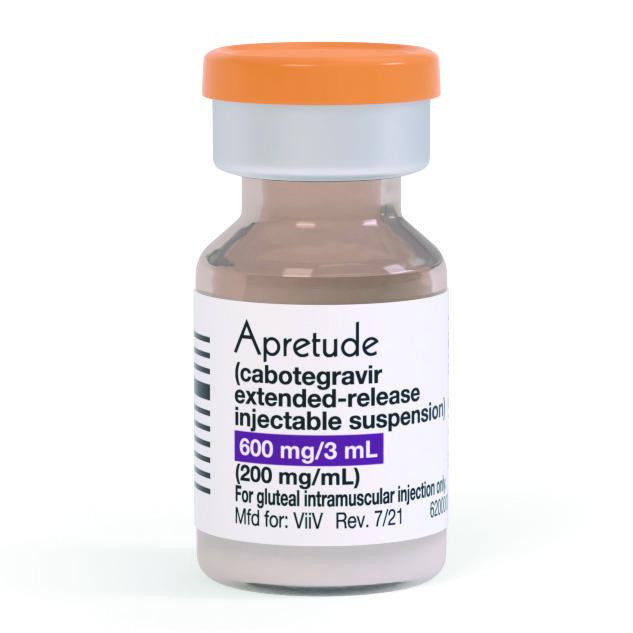
The U.S. Food and Drug Administration (FDA) approved a new form of HIV PrEP (pre-exposure prophylaxis—a medication used for disease prevention) on December 20.
It’s not just new. Some may see it as improved—in some ways, at least.
Apretude is taken as one long-acting injectable dose every two months. It is the third medication on the market for the prevention of HIV. The first two, Truvada and Descovy, are both taken as one tablet once daily.
“Today’s approval adds an important tool in the effort to end the HIV epidemic by providing the first option to prevent HIV that does not involve taking a daily pill,” said Debra Birnkrant, M.D., director of the Division of Antivirals in the FDA’s Center for Drug Evaluation and Research, in a press release. “This injection, given every two months, will be critical to addressing the HIV epidemic in the U.S., including helping high-risk individuals and certain groups where adherence to daily medication has been a major challenge or not a realistic option.”
The FDA went on to explain that according to the Centers for Disease Control and Prevention (CDC), “notable gains” in increasing PrEP use in the country have been made. Yet early data from 2020 show that only a quarter of the 1.2 million people for whom PrEP is recommended were prescribed it. Still, that compares with just 3% of the people for whom PrEP is recommended according to data back in 2015.
Noting that high levels of drug adherence are required for HIV PrEP use, the FDA said the new injectable could help increase prevention with PrEP, especially for some groups and for people who may be less likely to take daily medication correctly (be adherent).
Side effects occurring more often in people taking Apretude compared to Truvada in two clinical trials included injection site reactions, headache, pyrexia (fever), fatigue, back pain, myalgia (muscle aches and pain), and rash.
Apretude consists of the HIV medication cabotegravir (CAB). Cabotegravir is already on the market in the form of Cabenuva, which is made up of one shot of CAB and one shot of another long-acting medication, rilpivirine, taken once a month for HIV treatment.
For more information, see the upcoming Positively Aware annual HIV Drug Guide.
Welcome to the battle, Apretude.
Disappointing results lead to hold on HIV studies
A Phase 2 (early) study using the combination of two HIV drugs in development was stopped after decreases were observed in total lymphocytes and CD4+ T cell counts.
The two long-acting medications, islatravir along with a newer compound, MK-8507, were being studied in combination as a once-weekly complete oral regimen for HIV treatment.
Merck pharmaceutical made the announcement about its IMAGINE-DR (MK-8507-13) clinical trial in November. Merck will continue to monitor study participants. All were transitioned back to the original regimen they were on when they entered the study, Biktarvy. The participants had stable undetectable viral load when they entered the study and that did not change while they were in it.
In a conference call with community advocates, representatives of both the community and the company called the results disappointing. Merck spokespeople said the company is dedicated to continuing HIV treatment and prevention drug development safely, noting that new options are still needed.
The substantial decreases in immune system markers that were observed in IMAGINE-DR were dose dependent. The larger the dose of MK-8507, the greater the decrease.
The mean (average) decrease in total lymphocyte counts was 17% for the 100 mg dose of MK-8507, 26% for 200 mg, and 30% for 400 mg. For CD4+ T cell counts, the mean decrease was 11% for 100 mg, 23% for 200 mg, and 30% for 400 mg.
Looking at islatravir
As a result of the findings from IMAGINE-DR, Merck looked at all of its studies with islatravir, which are further along in development. Islatravir has oral and injectable formulations, and is being studied for both HIV prevention (PrEP) and HIV treatment.
At the time, Merck only found a total lymphocyte count decrease in one PrEP trial. The decreases were in the normal range and not considered clinically concerning. The mean decrease was 21% for the 60 mg dose of islatravir and 36% for the 120 mg dose. The people on a placebo saw an increase of 4%. All individuals, of course, were HIV-negative.
In both clinical trials, the control arms (the people who were not receiving the investigational medicine) saw no changes.
But as Michael Robertson, MD, exectuive director at Merck Research Laboratories, said, “Information is coming in fast and furious.”
On hold
Then in December, the U.S. Food and Drug Administration (FDA) put clinical holds on islatravir trials. Six studies were put on a full hold and seven were placed on partial holds.
With a full hold, participants no longer received islatravir and were put on their previous HIV regimen (what they were taking when they entered the study), or they were put on an HIV regimen or a PrEP medication that is already FDA approved and on the market.
With a partial hold, people were allowed to continue taking islatravir, but enrollment of new participants was stopped.
Doravirine plus islatravir moves forward
All studies of islatravir in combination with doravirine moved forward under a partial hold. Doravirine is an FDA-approved HIV medication from Merck, sold under the brand name Pifeltro. Merck announced in October that a Phase 3 (advanced) study of the two medications, taken together combined in a once-daily fixed-dose pill, had maintained undetectable viral load out to 48 weeks in people who had switched from a stable HIV regimen. In the ILLUMINATE SWITCH A study people were switching from any HIV regimen, while in the ILLUMINATE SWITCH B study they were switching over from Biktarvy.
As of press time, information was still coming in fast and furious.
Another person cured?
Another case of someone who may have been cured of HIV through their own immune system has been reported in the November 15 Annals of Internal Medicine. Read more about the case from science writers Ben Ryan for NBC News at nbcnews.com/health/health-news/womans-immune-system-possibly-cured-hiv-rcna5610 and Tim Murphy for TheBody.com at thebody.com/article/the-esperanza-patient-immune-system-cured-hiv.
In Chicago, a new program that helps recently incarcerated Black women living with HIV was announced by the AIDS Foundation of Chicago (AFC) during National Transgender Awareness Week, November 13–20. The Women Evolving Initiative offers critical reentry services, including economic opportunities, intensive case management, care coordination that includes behavioral health needs, and access to telehealth services.
“Studies show the imprisonment rate for Black women is more than 3.8 times the rate for White women and 2.4 times the rate of Hispanic women,” reported AFC. “Roughly 1.8 million Black women are released annually from local jails with the goal of rebuilding a life post-incarceration. Black women face much higher rates of homelessness and unemployment, and are much less likely to have a high school education.” The program serves all women, whether cisgender
or transgender.
For more information, call (312) 922-2322 or go to aidschicago.org.
HIV cases in children worldwide
On World AIDS Day (December 1), UNICEF reported that at least 300,000 children were newly infected with HIV in 2020, or one child every two minutes. Another 120,000 children died of AIDS-related causes, or one every five minutes. The organization noted that COVID has made things worse for women and children in regards to HIV. Read the UNICEF report at childrenandaids.org/2021-global-snapshot.

An updated National HIV/AIDS Strategy (NHAS) for 2022-2025 was released on World AIDS Day. It’s “designed to re-energize a whole-of-society response to the HIV epidemic” and provides a framework for the Biden Administration’s principles and priorities over the next four years, including a new focus on people aging with HIV and long-term survivors. Go to HIV.gov/topics/nhas.
In advance of World AIDS Day, White House Office of National AIDS Policy (ONAP) director Harold Phillips spoke about the day’s theme for 2021: “Ending the HIV Epidemic: Equitable Access, Everyone’s Voice.”
“[W]e need to pay attention to…the places in the country that we have health disparities, and where we have individuals and communities or geographic regions within our country that have a lot of HIV cases and HIV continues to increase in those areas as well. It means that we need to ensure that we leave no portions of our country and our populations behind.”
Learn more about the NHAS at HIV.gov/topics/nhas, and watch the video at bit.ly/HIV-strategy-2021-update.
Updated PrEP guidelines
The U.S. Centers for Disease Control and Prevention (CDC) issued the following statement as it presented updates to its HIV PrEP (pre-exposure prophylaxis—prevention) guidelines on December 8.
This statement is from Demetre C. Daskalakis, MD, MPH, Director of the Division of HIV Prevention in the National Center for HIV, Viral Hepatitis, STD, and TB Prevention.
Dr. Daskalakis says here, “PrEP is one of the most powerful tools we have to prevent HIV transmission. Expanding access to PrEP will be critical to ending the HIV epidemic in the United States.”

Today, the Centers for Disease Control and Prevention (CDC) published its updated Clinical Practice Guideline for Pre-exposure Prophylaxis for HIV Prevention and Clinical Providers Supplement. The updated guideline and supplement reflect the latest science and are intended to help physicians effectively prescribe all FDA-approved pre-exposure prophylaxis (PrEP) medications to patients and increase PrEP use among all people who could benefit.
The overall goals of the revisions are to update existing guidance using the current evidence base, incorporate recent and potential FDA actions on PrEP medications, clarify specific aspects of clinical care, and improve usability and increase implementation of the guideline.
Key revisions to the guideline include:
- A new recommendation for providers to inform all sexually active adults and adolescents about PrEP. This is intended to increase awareness of PrEP more broadly.
A recommendation that, in addition to taking a very brief history to identify persons with indications for PrEP, providers prescribe PrEP to anyone who requests it, even if they do not report specific HIV risk behaviors. This recommendation is intended to make PrEP available to people who may be apprehensive about sharing potentially stigmatized HIV risk behaviors with their provider.
A recommendation for F/TAF (Descovy) as an FDA-approved PrEP option for sexually active men and transgender women at risk of getting HIV, based on recent data showing its effectiveness for these populations.
A new section on prescribing bimonthly intramuscular injections of cabotegravir (CAB) for sexually active men and women who could benefit from PrEP, pending FDA data review and potential regulatory action.
Updated HIV testing recommendations that incorporate the latest and most effective methods for quickly detecting HIV infection among people using any PrEP medication.
Specifically, it includes a recommendation that providers now require the following for people who have taken oral PrEP in the last three months or who have received a CAB injection in the last 12 months:
- a positive antigen/antibody test and a detectable HIV-1 RNA test to confirm an HIV infection before transitioning the patient to an HIV treatment regimen; or
- a negative antigen/antibody test and an undetectable HIV-1 RNA test before confirming the absence of an HIV infection to continue prescribing PrEP.
Please note there are no changes to the guideline regarding populations for whom PrEP is recommended nor to the section of the guideline pertaining to recommended daily dosing regimens for oral PrEP. There are also no changes to the sections of the guideline regarding frequency of HIV and sexually transmitted infection (STI) testing for daily oral PrEP.
PrEP is one of the most powerful tools we have to prevent HIV transmission. Expanding access to PrEP will be critical to ending the HIV epidemic in the United States. CDC is committed to increasing the use of PrEP by funding high-impact HIV prevention programs for health departments and non-clinical and clinical community-based organizations around the country, including through the federal Ending the HIV Epidemic (EHE) in the U.S. initiative.
CDC programs are designed to increase PrEP awareness and demand by funding: local organizations to conduct community-based outreach to people who could benefit most including gay and bisexual men of color, people in the South, Black women, transgender women, and persons who inject drugs; education campaigns that increase awareness and combat stigma associated with PrEP use; and tools such as CDC’s PrEP Locator, which has information on public and private providers who offer PrEP.
CDC also aims to increase accessibility of PrEP through healthcare provider training, provider education campaigns, clinical guideline development, and by working with partners to offer PrEP and related services through primary care clinics, sexually transmitted disease (STD) clinics, TelePrEP services, pharmacies, and school-based health centers.
We have a once-in-a-generation opportunity to end the HIV epidemic in the United States, but to do so, we must maximize the use of effective prevention tools, such as PrEP. As the nation’s leading HIV prevention agency, CDC is committed to working with providers, partners, and communities across the country to increase the implementation of this updated guideline and increase the uptake of PrEP to reach our shared goal of reducing new HIV infections by 90% by 2030.
U=U website revamped
Prevention Access Campaign (PAC) has redesigned and expanded its website, making it into a clearinghouse for information and resources about U=U. Launched five years ago by PAC, U=U (undetectable equals untransmittable) is the message that a person living with HIV who is on antiretroviral treatment and has an undetectable viral cannot transmit the virus to someone through sex.
Among the website’s offerings:
- an interactive map to find U=U community partner organizations all over the world
- endorsements from a number of health organizations describing and explaining the significance of U=U, including UNAIDS, the World Health Organization, the Centers for Disease Control and Prevention, and the National Institutes of Health
- a link to Can’t Pass It On, a training course from the UK’s Terrence Higgins Trust to help health care professionals understand and discuss U=U with colleagues and patients
- an abstract article with companion video about how U=U improves the health and quality of life people living with HIV
Additional resources in other languages will be added, based on browser feedback. Go to preventionaccess.org.
Dr. Garofalo with plush versions of Fred
Fred Says donates over $200,000 to help young people around the world
On World AIDS Day 2021, the Chicago-based foundation Fred Says announced over $200,000 in donations to help young people around the world who are living with HIV or affected by it.
The awards were made in honor of Fred, the Yorkie who inspired the creation of the foundation. Fred passed away in August of last year, with his dad, Robert Garofalo, MD, at his side.
Fred Says awardees include TPAN, the non-profit HIV service organization that publishes Positively Aware; the Broadway Youth Center of Howard Brown Health; and the Ann and Robert H. Lurie Children’s Hospital of Chicago, where Dr. Garofalo is a pediatrician working with transgender youth. All three awardees are located in Chicago. Dr. Garofalo, who is a faculty member at Northwestern University’s Feinberg School of Medicine, helped found the community-based Broadway Youth Center in 2003 and has served on the TPAN board of directors since 2016.
“Fred may no longer be by my side, but he is forever a part of me. I am grateful for all that he has given, not just to me but to the world,” said Dr. Garofalo in a press release. “To that end, I have only just begun to fulfill the promise we made in 2013 to together make the world a better place for young people living with HIV. This year’s $200,000 [multi-year commitment to TPAN] in giving is by far the largest in Fred Says’ history and is the first of many that will honor my little man and help young people around the world who need just a little help to not just survive, but to thrive. Being able to support the work being done by TPAN, and our other awardees, with young people in the U.S. and abroad is a blessing and a gift in and of itself.”
TPAN serves young people from throughout the city in addition to adults. The award will specifically benefit the Tea Room, TPAN’s youth services and drop-in center, and Paws n’ Effect, the agency’s program linking people living with HIV with rescue pets.
Other donation recipients are the Callen-Lorde Health Center in New York, the Los Angeles Children’s Hospital Adolescent HIV Program, Birmingham AIDS Outreach in Alabama, Children’s Hospital of Philadelphia’s Adolescent Initiative, Advocates for Youth, the University of Ibadan in Nigeria, and the Desmond Tutu HIV Foundation Youth Center in South Africa.
Fred’s story has appeared in the pages of Positively Aware several times, and he appeared with Dr. Garofalo on last year’s cover of the January+February issue. His death was devastating, but his legacy lives on.
Correction
In “Transforming Power” in the November + December 2021 issue, Lisa Spedalle, RN, works with the Visiting Nurse Service of New York (VNSNY), not the Visiting Nurses Society, which is a different group. Positively Aware apologizes to Spedalle and VNSNY for the error.